42 examples of exempt human specimens
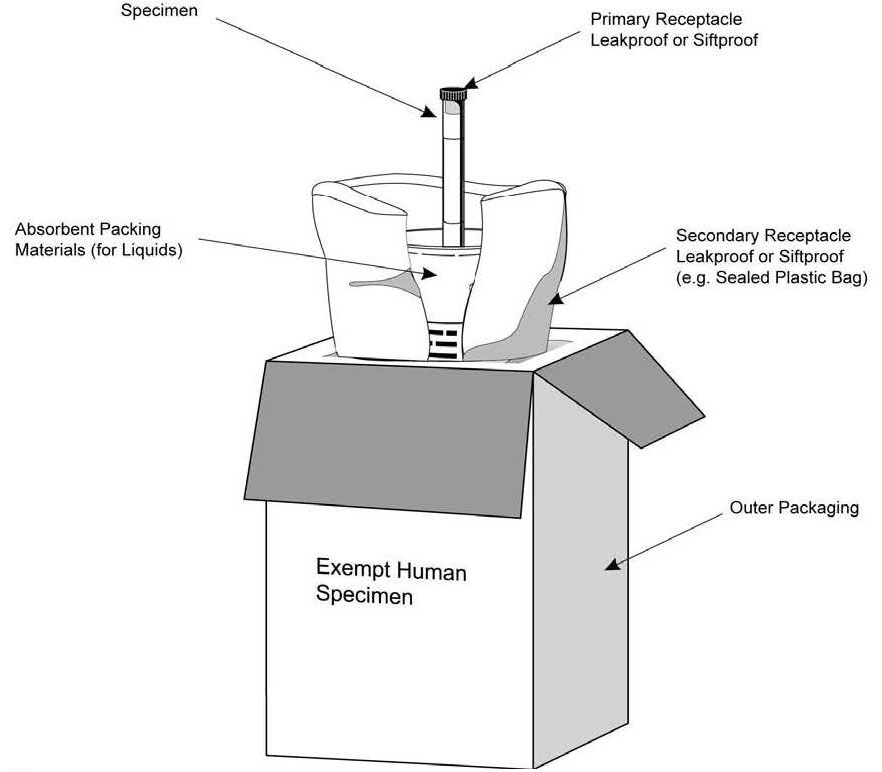
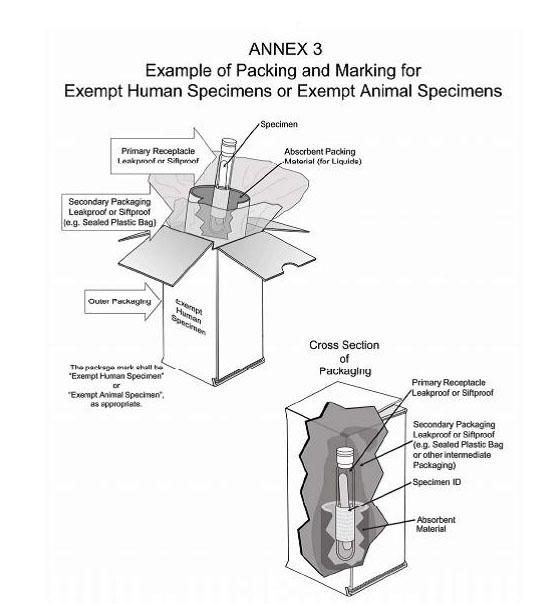
Exempt Animal or Human Specimens | Environment, Health and Safety "Exempt Animal Specimen" The package must consist of three components: Leek-proof primary receptacle Leak-proof secondary packaging For liquids, absorbent material sufficient to absorb the entire contents must place between the primary receptacle and the secondary packaging Marking and Labelling for Exempt Human Specimens or Biological ... 3.3) Exempt Human Specimens · Blood or urine tests to monitor cholesterol/glucose/hormone · Liver or kidney function for patients with non-infectious disease ...
Laboratory Shipping Protocols - National Institutes of Health Examples of specimens which may be transported as a patient specimen for which there is a minimal likelihood that pathogens are present include blood or urine tests to monitor cholesterol levels, blood glucose levels, hormone levels, or prostate specific antigens (PSA); tests required to monitor organ function such as heart, liver or kidney …
Examples of exempt human specimens
PDF Office of Research Protections Human Research Protection Office Data ... research. A regulatory determination of "research not involving human subjects" or "exempt human subjects research" will not suffice. I. Specimen Research that Does Not Require HRPO Review Prior to Implementation. The exceptions described in Sections I.A., B., and C. of this guidance apply only to the specified PDF Regulated and Non-regulated Biological Materials Exempt Human and Animal specimens are considered dangerous goods by IATA until they meet three criteria: 1. A professional determination has been made that the material has a "minimal likelihood:" of containing any pathogens (Need Appropriate Training to meet this criteria) Exempt Research: Guidance: Human Subjects & Institutional Review Boards ... This is a list of examples of research that may be exempt and additional information on exempt categories. ... Exempt research is still subject to human subjects review, but is not required to comply with the same requirements which are applied to expedited and full board research. ... pathological specimens, or diagnostic specimens if: These ...
Examples of exempt human specimens. IATA Dangerous Goods Regulations | IATA Requirements | Therapak Examples of those tests are: blood or urine for cholesterol levels, hormone levels, prostate specific antigens (PSA), tests to monitor organ function (heart, liver, kidney), tests conducted for insurance or employment, pregnancy, biopsies for cancer, drug or alcohol presence testing. Shipping Biological Substances FAQs | UPS - United States What is an Exempt Human Specimen or Exempt Animal Specimen? "Patient specimens for which there is minimal likelihood that pathogens are present." (Dangerous Goods Regulations, 3.6.2.2.3.8) Refer to 49 USC § 173.134 for additional information regarding domestic shipping. How to Pack Specimens Correctly - Karolinska Institutet Regulations for the transport of infectious waste are not covered in How to Pack Specimens Correctly. Exempt human/animal specimen. Samples taken from humans in ... Exempt patient specimens - Home un3373.it They are collected directly from humans or animals and there is minimal likelihood that pathogens are present. An element of professional judgment is required ...
Shipping infectious substances - Transport Canada Patient specimens are those collected directly from humans or animals and include, for example, excreta, blood and its components, tissue and tissue fluids swabs, and body parts being transported for purposes such as research, diagnosis, investigational activities, disease treatment and prevention. Cultures versus patient specimens Coded Private Information or Specimens Use in Research, Guidance (2008) The following are examples of private information or specimens that will be collected in the future for purposes other than the currently proposed research: (1) medical records; and (2) ongoing collection of specimens for a tissue repository. Frequently Shipped Biological Material and Proper Classification Exempt patient specimens include: Biopsies. Dried blood spots. Fecal occult blood screening test. Specimens (blood, urine, tissue) being sent for antibody detection, organ function or therapeutic drug monitoring, pregnancy, drug, insurance, or employment test purposes, etc. Tissues for transplant. Research Using Human Subjects - National Institute of Allergy and ... For example, this would include research on living persons using: Bodily materials such as cells, blood, urine, tissues, organs, hair or nail clippings, even if collected by others. Residual diagnostic specimens, including those from routine patient care, that are kept for research rather than discarded.
Biospecimens: Areas of Research: Guidance: Human Subjects ... In addition, the FDA requires IRB review for the use of de-identified human specimens in clinical investigations of medical devices when the research may generate or collect data that may be submitted to the FDA for review. Examples of biospecimen research requiring IRB review (exempt, expedited, or full board review): PDF Preparing Shipments of Exempt Biological Specimens Examples of Exempt Human/Animal Specimens include: • Biopsies • Dried blood spots • U rine • Fecal samples • Tissues • B lood* • Cells *Blood taken from an otherwise healthy patient that is not suspected to contain an infectious pathogen from Category A or Category B biological substances may be exempt from shipping regulations. ... PDF Guidelines for Human Biospecimen - National Institutes of Health Certain human subjects research with biospecimens may be exempt from the requirement for initial and continuing IRB review and approval if the research involves temporary access to identifiable biospecimens, but the research data is recorded by the researcher in a de-identified manner. Exempt Human Specimen / Exempt Animal Specimen ... Examples of specimens which may be transported as Exempt include: the blood or urine tests to monitor cholesterol levels, blood glucose levels, hormone levels, or prostate specific antigens (PSA); tests required to monitor organ functions such as heart, liver, or kidney function for humans or animals with noninfectious diseases, or therapeutic …
How To Ship Exempt Human & Animal Specimen - shipmercury.com Some examples of Exempt Patient/Human/Animal Specimens: Routine testing of blood or urine tests ordered for a medical examination Cholesterol, Blood Glucose Insurance or employment tests DNA tests Pregnancy tests Tests done for other than testing for the presence of pathogens
PDF Guidance Doc Infectious Substances - ICAO Examples of specimens which may be transported as a patient specimen for which there is a minimal likelihood that pathogens are present include blood or urine tests to monitor cholesterol levels, blood ... Addendum No. 2, dated 30/6/05, the 2005-2006 ICAO Technical Instructions require exempt human or animal specimens to be packaged and marked ...
Shipping Checklist for Exempt Human/Animal Specimens Jan 1, 2021 ... Are the shipper and consignee (recipient) names and addresses legible, complete, and clearly visible? Are the words “Exempt Human Specimens” or ...
Research Using Human Biological Specimens Specifically, exempt category #4 applies to research that involves the collection or study of existing * data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available ** or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or …
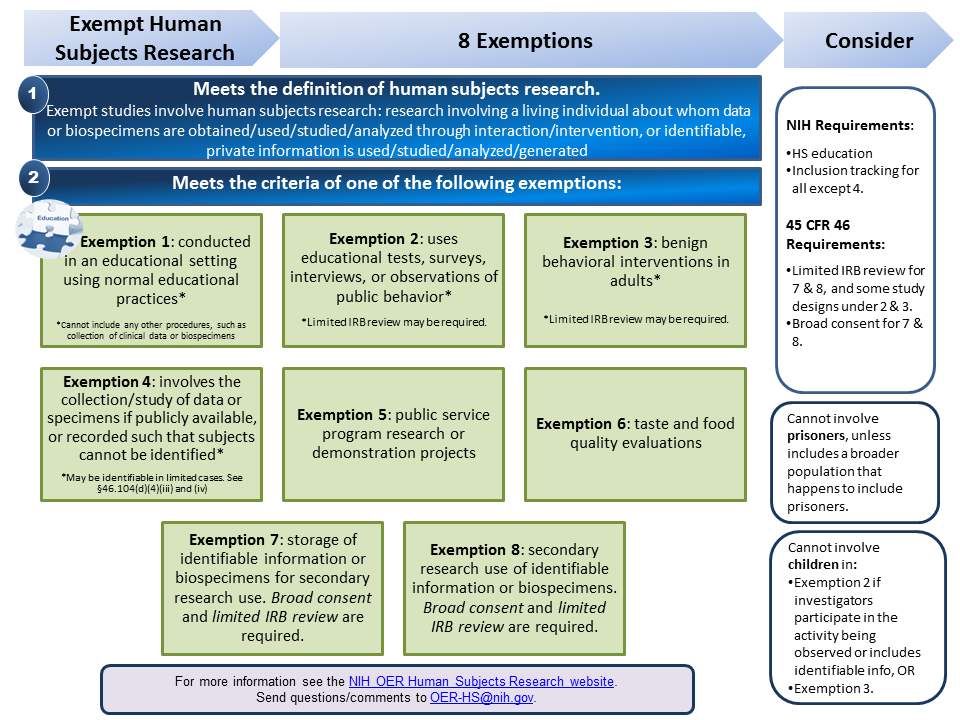
Definition of Human Subjects Research | grants.nih.gov It summarizes Exemptions 1, 2, 3, 4, 5, 6, 7 and 8, providing basic definitions, examples of studies that meet and do not meet the criteria of the exemption, and aspects one must consider when engaged in exempt or non-exempt human subjects research. Research Involving Private Information or Biospecimens Flowchart
PDF Step 3: Packing Category A and B and Exempt Human and Exempt ... - CDC 2Exempt Human Specimen or Exempt Animal Specimen Packaging Leakproof/primary receptacle Outer packagingstrong enough forcapacity, mass, and intended use Leakproof/ siftproofsecondary packaging Biohazard symbol(USPS only) and consignee information Documentation Exempt Human Specimen or Exempt Specimen
Exempt Research: Guidance: Human Subjects & Institutional Review Boards ... This is a list of examples of research that may be exempt and additional information on exempt categories. ... Exempt research is still subject to human subjects review, but is not required to comply with the same requirements which are applied to expedited and full board research. ... pathological specimens, or diagnostic specimens if: These ...
PDF Regulated and Non-regulated Biological Materials Exempt Human and Animal specimens are considered dangerous goods by IATA until they meet three criteria: 1. A professional determination has been made that the material has a "minimal likelihood:" of containing any pathogens (Need Appropriate Training to meet this criteria)
PDF Office of Research Protections Human Research Protection Office Data ... research. A regulatory determination of "research not involving human subjects" or "exempt human subjects research" will not suffice. I. Specimen Research that Does Not Require HRPO Review Prior to Implementation. The exceptions described in Sections I.A., B., and C. of this guidance apply only to the specified










Post a Comment for "42 examples of exempt human specimens"